Skin stem cells during development, homeostasis and repair
The skin epidermis is a stratified epithelium composed of the interfollicular epidermis, which constitutes the skin barrier, and different appendages such as the hair follicles with their associated sebaceous glands and the sweat glands. The interfollicular epidermis is composed of one single inner layer of proliferative cells, called the basal layer, and several suprabasal layers containing postmitotic terminally differentiated cells.
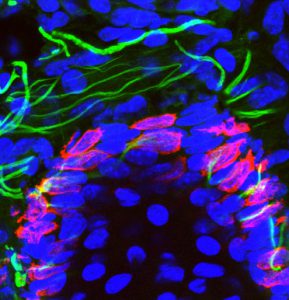
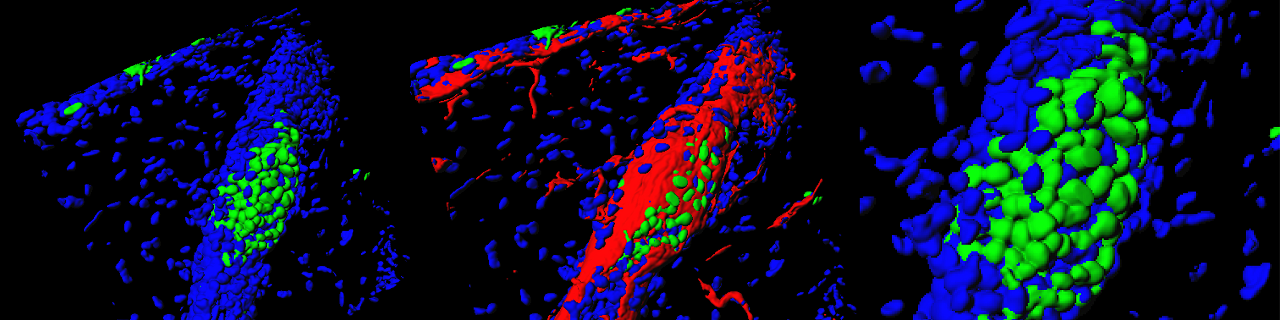
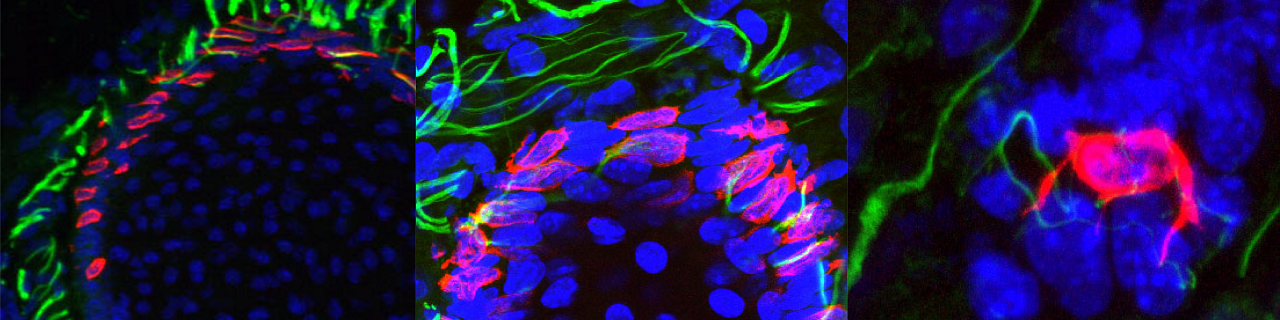
Merkel cells are neuroendocrine cells located in the epidermis that mediate touch sensation. Merkel cells were thought to originate from neural crest cells. Using lineage tracing, our lab demonstrated that Merkel cells did not arise from neural crest but rather originate from epidermal stem cells by an Athoh1/Math1 dependent mechanism (Journal Cell Biology 2009).
During adult life, when the animal and the skin reaches its final size, the interfollicular epidermis undergoes a process of continual cellular turnover throughout life to replace the damaged and dead cells that are constantly shed from skin the surface. To maintain the size of tissue constant, the number of cell division must exactly compensate the number of cell lost.
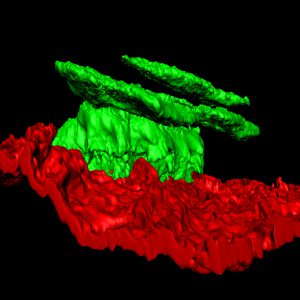
Our lab is interested in understanding the mechanisms that regulate epidermal development, homeostasis and repair. Using quantitative clonal analysis in the adult mouse epidermis our group has recently demonstrated the existence of a hierarchical organization of stem cells and progenitors that sustains epidermal homeostasis and repair. We demonstrated the existence of a population of long-lived slow cycling stem cells, that co-exist with a population of progenitors that asymmetrically divide at the population level to sustain the renewal of the epidermis. During wound healing only stem cells but not progenitors contribute to the long-term repair of the skin epidermis (Nature 2012 and Nature 2016).
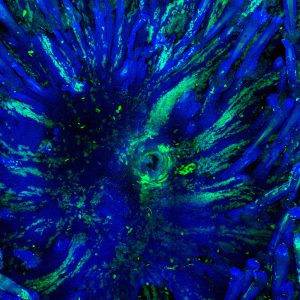
Using quantitative clonal analysis, we unraveled the clonal dynamics of stem cells and progenitors during wound healing. We uncovered and molecularly characterized two distinct cellular compartments, a proliferative center and migrating front that are essential for harmonious wound healing (Nature Communications 2017).
People
Anaïs Bauduin, PhD student
Mélanie Liagre, technician
Selected publications
Epidermal homeostasis: a balancing act of stem cells in the skin. Blanpain C & Fuchs E. Nature Reviews Molecular and Cell Biology 2009. (3): 207-17.
Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. Van Keymeulen A, Mascre G, Kass Youseff K, Harel I, Michaux C, De Geest N, Spalski C, Achouri Y, Bloch W, Hassan BA and Blanpain C. Journal of Cell Biology 2009, 187:91-100. Cover article accompanied by an editorial highlight
Distinct contribution of stem and progenitor cells to epidermal maintenance. Mascré G, Dekoninck S, Drogat B, Youssef Kk, Brohée S, Sotiropoulou P, Simons BD, and Blanpain C. Nature. 2012 Sep 13;489(7415):257-62. Highlighted by a preview and news and views in Nature and Nature Reviews Molecular and Cellular Biology.
Defining the clonal dynamics leading to mouse skin tumor initiation. Sánchez-Danés A, Hannezo E, Larsimont JC, Liagre M, Youssef KK, Simons B, Blanpain C. Nature. 2016 Aug 18;536(7616):298-303. Highlighted by a preview in Cancer Discovery
Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascré M, Simons B, Blanpain C. Nature Communications 2017 Mar 1;8:14684.
Defining the Design Principles of Skin Epidermis Postnatal Growth. Dekoninck S, Hannezo E, Sifrim A, Miroshnikova YA, Aragona M, Malfait M, Gargouri S, de Neunheuser C, Dubois C, Voet T, Wickström SA, Simons BD, Blanpain C. Cell. 2020 Apr 30;181(3):604-620.e22. doi: 10.1016/j.cell.2020.03.015. Epub 2020 Apr 6. Cover article
Mechanisms of stretch-mediated skin expansion at single-cell resolution. Aragona M, Sifrim A, Malfait M, Song Y, Van Herck J, Dekoninck S, Gargouri S, Lapouge G, Swedlund B, Dubois C, Baatsen P, Vints K, Han S, Tissir F, Voet T, Simons BD, Blanpain C. Nature. 2020 Aug;584(7820):268-273. doi: 10.1038/s41586-020-2555-7. Highlighted by a news and views and a podcast in Nature
Dynamic regulation of tissue fluidity controls skin repair during wound healing. Sarate RM, Hochstetter J, Valet M, Hallou A, Song Y, Bansaccal N, Ligare M, Aragona M, Engelman D, Bauduin A, Campàs O, Simons BD, Blanpain C.
Cell. 2024 Aug 11:S0092-8674(24)00825-0. doi: 10.1016/j.cell.2024.07.031.
Survivin Promotes Stem Cell Competence for Skin Cancer Initiation.
Canato S, Sarate R, Carvalho-Marques S, Maia Soares R, Song Y, Monteiro-Ferreira S, Vieugué P, Liagre M, Grossi G, Cardoso E, Dubois C, Conway EM, Schenone S, Sánchez-Danés A, Blanpain C.
Cancer Discov. 2025 Feb 7;15(2):427-443. doi: 10.1158/2159-8290.CD-24-0263.