Mechanisms regulating tumor heterogeneity
The importance of tumor heterogeneity and its potential impact for personalized medicine is widely recognized. Different mechanisms including genetic and non-genetic factors such as the existence of different populations of tumor cells including cancer stem cells to regulate tumor heterogeneity.
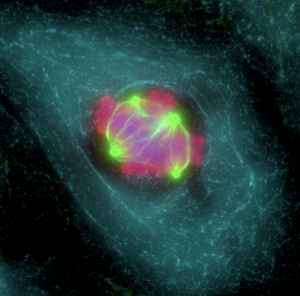 We have assessed the importance of genetic determinants of tumor heterogeneity using next generation sequencing of tumor cells. We demonstrated the importance of the mutation landscape in regulating skin squamous cell carcinoma (SCC) heterogeneity (Nature Medicine 2015). It remains a matter of intense debate to whether aneuploidy is the cause or the consequence of tumorigenesis. We demonstrated that aneuploidy is associated with malignant transition (Nature Medicine 2015) and can promote tumor development only in p53 deficient context, resolving a long-standing debate in the field (Nature Cell Biology 2016). Cancer stem cell potential is usually assessed by the ability of cancer cells to propagate the tumor upon transplantation into immunodeficient mice. Our lab has been pioneered to develop new genetic approaches to assess the fate of tumor cells within their natural environment.
We have assessed the importance of genetic determinants of tumor heterogeneity using next generation sequencing of tumor cells. We demonstrated the importance of the mutation landscape in regulating skin squamous cell carcinoma (SCC) heterogeneity (Nature Medicine 2015). It remains a matter of intense debate to whether aneuploidy is the cause or the consequence of tumorigenesis. We demonstrated that aneuploidy is associated with malignant transition (Nature Medicine 2015) and can promote tumor development only in p53 deficient context, resolving a long-standing debate in the field (Nature Cell Biology 2016). Cancer stem cell potential is usually assessed by the ability of cancer cells to propagate the tumor upon transplantation into immunodeficient mice. Our lab has been pioneered to develop new genetic approaches to assess the fate of tumor cells within their natural environment.
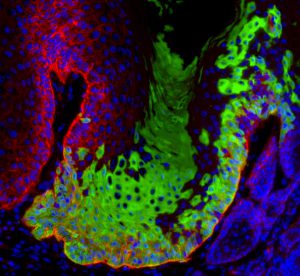 We have performed for the first time clonal analysis to unravel the mode of tumour growth and provide the first experimental evidence for the existence of cancer stem cells during primary tumour growth in vivo (Nature 2012). We also used lineage ablation to demonstrate the critical role of a small population of cancer stem cells expressing Sox2 that are responsible for tumor maintenance in squamous cell carcinoma (Nature 2014). These two new methods become the gold standard to assess cancer stem cells in vivo.
We have performed for the first time clonal analysis to unravel the mode of tumour growth and provide the first experimental evidence for the existence of cancer stem cells during primary tumour growth in vivo (Nature 2012). We also used lineage ablation to demonstrate the critical role of a small population of cancer stem cells expressing Sox2 that are responsible for tumor maintenance in squamous cell carcinoma (Nature 2014). These two new methods become the gold standard to assess cancer stem cells in vivo.
We uncovered a dual role for VEGF in regulating cancer stem cells. The secretion of VEGF by tumor cells stimulates neoangiogenesis, which create a vascular niche for cancer stem cells. VEGF also acts directly on tumor cells in an autocrine loop through a Nrp1 dependent mechanism to promote cancer stem cell renewal and tumour growth (Nature 2011). We have demonstrated the critical role of the transcription factors Sox2 and Twist1 in regulating the renewal and differentiation of cancer stem cells (Nature 2014 and Cell Stem Cell 2015).
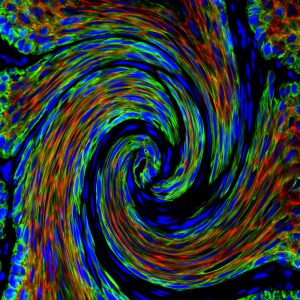
By screening a large panel of cell surface markers and performing single cell RNA sequencing, we have identified different tumor states associated with different degree of epithelial to mesenchymal transition (EMT) including intermediate hybrid states in different mouse and human cancers and identified populations of EMT states associated with increased metastatic potential (Nature 2018).
Tumor heterogeneity was also proposed to regulate response to therapy. We identified a population of tumour cells that persists despite the administration of smoothened inhibitor and is responsible for basal cell carcinoma relapse after therapy. We discovered that inhibition of Wnt signalling combined with smoothened inhibitor leads to the eradication of the persisting tumor cells, showing that dual Wnt and hedgehog inhibition is a clinically relevant strategy for overcoming tumour relapse in patients with basal cell carcinoma (Nature 2018).
FAT1 is one of the most frequently mutated genes in human cancers. However, the role and the molecular mechanisms by which FAT1 mutations control tumour initiation
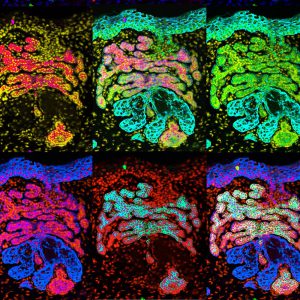
and progression are poorly understood. We found that Fat1 deletion accelerates tumour initiation and malignant progression and promotes a hybrid EMT state in mouse and human squamous cell carcinomas. We demonstrated that loss of function of FAT1 activates a CAMK2-CD44-SRC axis that promotes YAP1 nuclear translocation and ZEB1 expression that stimulates the mesenchymal state. This comprehensive analysis identified drug resistance and vulnerabilities in FAT1-deficient tumours, which have important implications for cancer therapy (Pastushenko Nature 2021).
The nongenetic mechanisms required to sustain malignant tumor state are poorly understood. Using genetic gain of function and loss of function in vivo, we showed that NR2F2 is essential for promoting the malignant tumor state by promoting tumor cell proliferation, EMT and invasive features, while repressing tumor differentiation in mouse and human SCC (Mauri Nature Cancer 2021).
The resistance of cancer cells to therapy is responsible for the death of most patients with cancer. EMT has been associated with resistance to therapy in different cancer cells. However, the mechanisms by which EMT mediates resistance to therapy remain poorly understood. Using a mouse model of skin SCCs undergoing spontaneous EMT, we found that EMT tumour cells are highly resistant to a wide range of anti-cancer therapies. Using gain and loss of function studies, we found that RHOJ-a small GTPase preferentially expressed in EMT cancer cells-controls resistance to chemotherapy by promoting tumour cells to rapidly repair DNA lesions induced by chemotherapy, uncovering the mechanisms by which RHOJ acts as a key regulator of EMT-associated resistance to chemotherapy (Debaugnies Nature 2023).
Up to now, there was no pharmacological approaches targeting EMT in cancer. We found that cancer cells presenting EMT express high levels of Netrin-1 and its receptor UNC5B. We showed that the administration of a therapeutic antibody targeting the interaction between Netrin-1 and Unc5b inhibits EMT, reduces their ability to give rise to metastases and sensitizes tumor cells to chemotherapy. Anti-Netrin-1 antibody decreased EMT in A549 human cancer cell line (Lengrand Nature 2023). In a companion paper published back to back in Nature, in collaboration with the University of Lyon and Nétris Pharma, we showed that the administration of the anti-Netrin-1 antibody inhibits EMT in cancer patients, demonstrating for the first time that EMT can be pharmacologically targeted in pre-clinical models and in patients with cancer (Cassier Nature 2023).
People
Kevin Bévant, postdoctoral researcher
Anqi Dong, postdoctoral researcher
Sebastiaan Vanuytven, postdoctoral researcher
Qiang He, postdoctoral researcher
Gabriel Windels, PhD student
Andrea Perez Gonzalez, PhD student
Monica Jordan Pirla, PhD student
Julie Sarrand, PhD student
Sara Llorens Carmona, PhD student
Catherine Paulissen, technician
Virginie Moers, technician
Sophie Lemaire, technician
Samuel Scozzaro, technician
Jérémy Blondeau, technician
Lieselot Van de Putte, technician
Christophe Riguelle, technician
Selected publications
The vascular niche and a VEGF/Nrp1 loop regulates the initiation and stemness of skin tumours. Beck B , Driessens G, Goossens G, Kass Youssef K, Loges S, Caauwe A, Kuchnio A, Sotiropoulou PA, Candi A, Mascre M, Haigh JJ, Carmeliet P, and Blanpain C. Nature 2011 Oct 9;479(7372):189-93. Highlighted by a preview and news and views in Nature and Nature Reviews Molecular and Cellular Biology.
Defining the mode of tumour growth by clonal analysis. Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Nature. 2012 Aug 23;488(7412):527-30. Highlighted by a preview and news and views in Nature, Science, Nature Biotech, Nature Methods, Nature Reviews Cancer, and Science and Business exchange and the popular press around the world.
Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness. Lapouge G, Beck B, Nassar D, Dubois C, Dekoninck S, Blanpain C. EMBO J. 2012 Nov 27;31(24):4563-75.
Unravelling cancer stem cell potential. Beck B, Blanpain C. Nat Rev Cancer 2013-13, 727–738.
Sox2 controls tumour initiation and cancer stem cell functions in squamous cell carcinoma. Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Lemercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohée S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B & Blanpain C. Nature 2014 Jul 10;511(7508):246-50. Highlighted in Cancer Cell and EMBOJ.
Different Twist1 levels regulates tumor intiation, stemness and progression
Beck B, Lapouge G, Drogat B, Desaedelaere K, Delafaille S, Willekens K, Marine J.C. and Blanpain C. Cell Stem Cell 2015 Jan 8;16(1):67-79.
Cover article
Genomic landscape of carcinogen and genetically-induced mouse skin squamous cell carcinoma. Nassar D, Latil M, Boeckx B, Lambrechts D, Blanpain C. Nat Med. 2015 Aug;21(8):946-54.
Toward understanding and exploiting tumor heterogeneity. Alizadeh AA, Aranda V, Bardelli A, Blanpain C, Bock C, Borowski C, Caldas C, Califano A, Doherty M, Elsner M, Esteller M, Fitzgerald R, Korbel JO, Lichter P, Mason CE, Navin N, Pe’er D, Polyak K, Roberts CW, Siu L, Snyder A, Stower H, Swanton C, Verhaak RG, Zenklusen JC, Zuber J, Zucman-Rossi J. Nat Med. 2015 Aug;21(8):846-53. doi: 10.1038/nm.3915.
Transient PLK4 overexpression accelerates tumorigenesis in p53-deficient epidermis. Sercin O, Larsimont JC, Karambelas AE,Marthiens V, Moers V, Boeckx B, Le Mercier M, Lambrechts D, Basto R, Blanpain C. Nature Cell Biology, 2016 Jan;18(1):100-10.
Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Nassar D, Blanpain C. Annu Rev Pathol. 2016 May 23;11:47-76.
Identification of the tumour transition states occurring during EMT Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Renvenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S, De Clercq S, Minguijon E, Cédric Balsat C, Sokolow Y, Dubois C, de Cock F, Scozzaro S, Sopena F, Lanas A, D’Haene N, Salmon I, Marine JC, Voet T, Sotiropoulou P and Blanpain C. Nature. 2018 Apr;556(7702):463-468. Highlighted by a news and views in Nature.
A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Sánchez-Danés A, Larsimont JC, Liagre M, Muñoz-Couselo E, Lapouge G, Brisebarre A, Dubois C, Suppa M, Sukumaran V, Del Marmol V, Tabernero J, Blanpain C. Nature. 2018 Oct;562(7727):434-438.
Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Pastushenko I, Mauri F, Song Y, de Cock F, Meeusen B, Swedlund B, Impens F, Van Haver D, Opitz M, Thery M, Bareche Y, Lapouge G, Vermeersch M, Van Eycke YR, Balsat C, Decaestecker C, Sokolow Y, Hassid S, Perez-Bustillo A, Agreda-Moreno B, Rios-Buceta L, Jaen P, Redondo P, Sieira-Gil R, Millan-Cayetano JF, Sanmatrtin O, D’Haene N, Moers V, Rozzi M, Blondeau J, Lemaire S, Scozzaro S, Janssens V, De Troya M, Dubois C, Pérez-Morga D, Salmon I, Sotiriou C, Helmbacher F, Blanpain C. Nature. 2021 Jan;589(7842):448-455.
NR2F2 controls malignant squamous cell carcinoma state by promoting stemness and invasion and repressing differentiation. Mauri M, Schepkens C, Lapouge G, Drogat B, Song Y, Pastushenko I, Rorive S, Blondeau J, Golstein S, Bareche Y, Miglianico M, Nkusi E, Rozzi M, Moers M, Brisebarre A, Raphael M, Dubois C, Allard J, Durdu B, Ribeiro F, Sotiriou C, Salmon I, Vakili J & Blanpain C. Nature Cancer. 2021 Nov;2(11):1152–1169.
RHOJ controls EMT-associated resistance to chemotherapy. Debaugnies M, Rodríguez-Acebes S, Blondeau J, Parent MA, Zocco M, Song Y, de Maertelaer V, Moers V, Latil M, Dubois C, Coulonval K, Impens F, Van Haver D, Dufour S, Uemura A, Sotiropoulou PA, Méndez J, Blanpain C. Nature. 2023 Apr;616(7955):168-175. Highlighted by a news and views in Nature and in Nature Review Cancer
Pharmacological targeting of netrin-1 inhibits EMT in cancer. Lengrand J, Pastushenko I, Vanuytven S, Song Y, Venet D, Sarate RM, Bellina M, Moers V, Boinet A, Sifrim A, Rama N, Ducarouge B, Van Herck J, Dubois C, Scozzaro S, Lemaire S, Gieskes S, Bonni S, Collin A, Braissand N, Allard J, Zindy E, Decaestecker C, Sotiriou C, Salmon I, Mehlen P, Voet T, Bernet A, Blanpain C. Nature. 2023 Aug;620(7973):402-408. Highlighted in Nature Review Drug Discovery
Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Cassier PA, Navaridas R, Bellina M, Rama N, Ducarouge B, Hernandez-Vargas H, Delord JP, Lengrand J, Paradisi A, Fattet L, Garin G, Gheit H, Dalban C, Pastushenko I, Neves D, Jelin R, Gadot N, Braissand N, Léon S, Degletagne C, Matias-Guiu X, Devouassoux-Shisheboran M, Mery-Lamarche E, Allard J, Zindy E, Decaestecker C, Salmon I, Perol D, Dolcet X, Ray-Coquard I,Blanpain C*, Bernet A*, Mehlen P* (*last co-authors). Nature. 2023 Aug;620(7973):409-416. Highlighted in Nature Review Drug Discovery


